WHAT IS RADIATION?
Radiation is the process by which energy is emitted as either particles or waves. Broadly, it can take the form of sound, heat, or light. However, most people generally use it to refer to radiation from electromagnetic waves, ranging from radio waves, through the visible light spectrum, and up through to gamma waves.

ATOMS AND THEIR PARTS
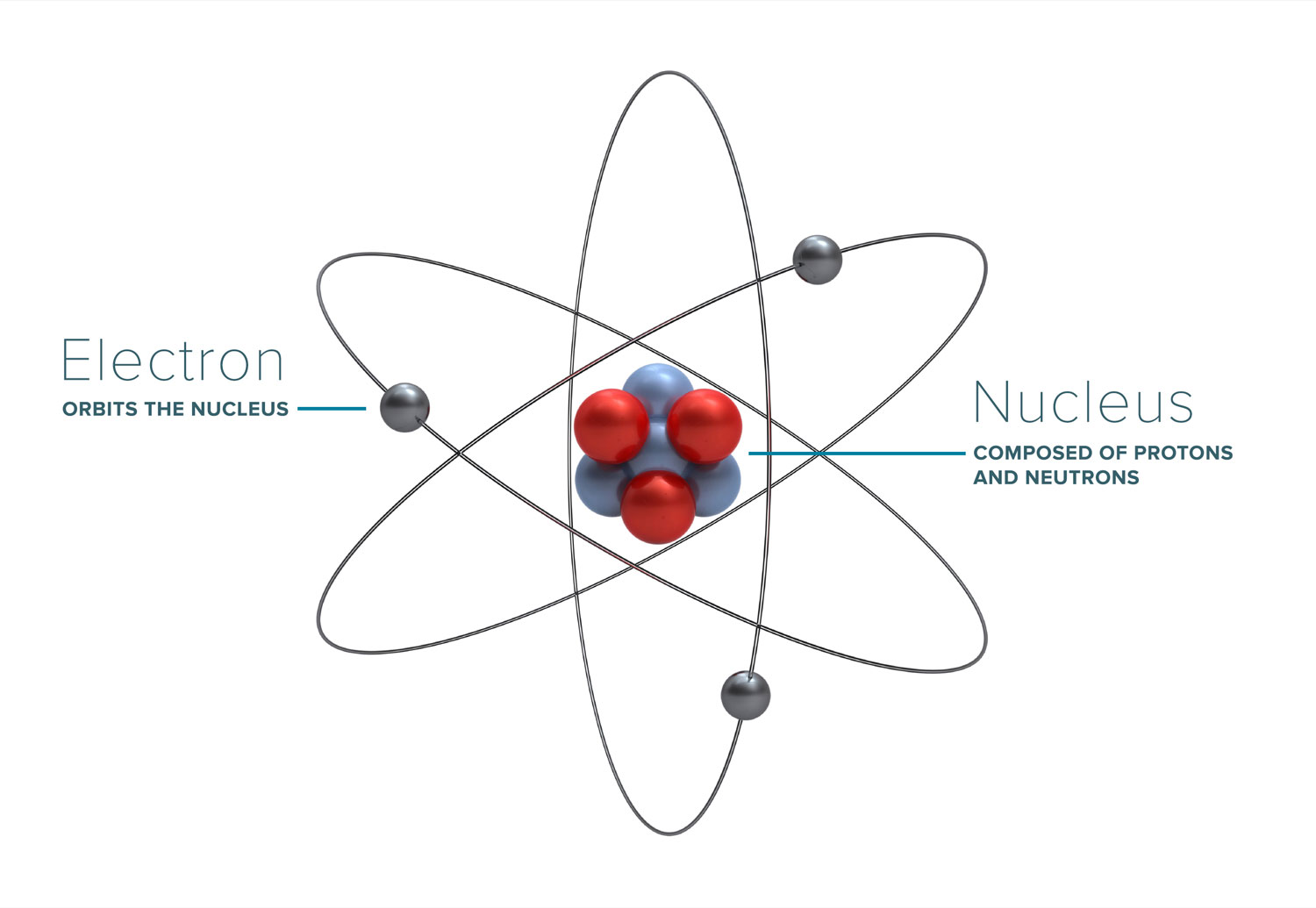
Most of the discussion about radiation, how it works, and what its effects are boil down to the interaction of radiation with atoms (and molecules) that it comes into contact with. Atoms form the basic building blocks of all matter. They consist of a nucleus, made of positively-charged protons (and sometimes neutrally-charged neutrons), and an outer cloud of electrons, which have a negative charge. The positive charge of a single proton is equal to the negative charge of a single electron.
Protons and neutrons have a relatively large size and atomic weight, whereas electrons are extremely small and light by comparison. Due to the nature of opposite charges attracting, atoms tend to have an equal number of protons and electrons, leaving the atom as a whole having a net charge of zero. However, if the atom either loses or gains an electron, it becomes an ion, and carries a charge.
It will seek bonds with other charged particles in order to regain a neutral balance, potentially leading to new molecules being formed.
IONIZING VS NON-IONIZING RADIATION
Radiation is generally classified ionizing or non-ionizing, based on whether it has enough energy to knock electrons off atoms that it interacts with, as well as being able to do lower-energy damage such as breaking chemical bonds in molecules. Ionizing radiation, which is caused by unstable atoms giving off energy to reach a more stable state, is more of a health threat to humans because it involves changing the basic makeup of atoms in cells, and more specifically the DNA molecules inside of cells. It does, of course, take a very strong dose of radiation to substantially damage a cell’s structure, as there can be trillions of atoms in a single cell.
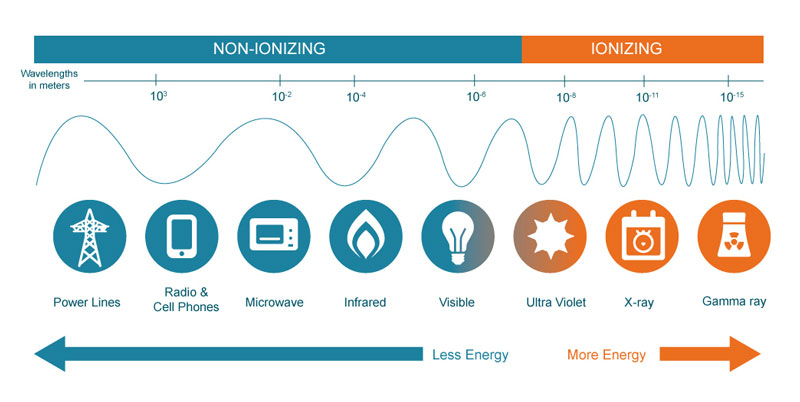
Most non-ionizing radiation, such as radio and microwave energy, is considered harmful only to the extent of the amount of heat energy it transfers to whatever it hits. This is, in fact, the way that microwaves cook food. UV light is unique in that while it is non-ionizing, it does have the capacity to cause harmful effects similar to what ionizing radiation can create, such as an increased risk of cancer due to damage to DNA molecules.
HOW IS RADIATION MEASURED?
The radioactivity of a substance, or how “active” it is radioactively, is measured in either curies (Ci) or Becquerel’s (Bq). Both are measures of the number of decays per second, or how often an atom in a given sample will undergo radioactive decay and give off a particle or photon of radiation. The curie (1 Ci equals about 37,000,000,000 decays per second) is named after Marie and Pierre Curie, and is equal to roughly the activity of one gram of radium, which they studied. The Becquerel is the SI unit for radioactivity. One Bq equals one decay per second. The Bq is the SI unit, though the curie remains widely used throughout the US in both government and industry.
RADIATION SAFETY
The instadose+ dosimeter helps Radiation Safety Officers (RSO) save time managing dosimetry programs (Know more of dosimeter)